Breathtaking Info About Do Electrons Revolve Clockwise Or Anticlockwise

The Quantum Numbers +1/2 And 1/2 For Electron S... Physics
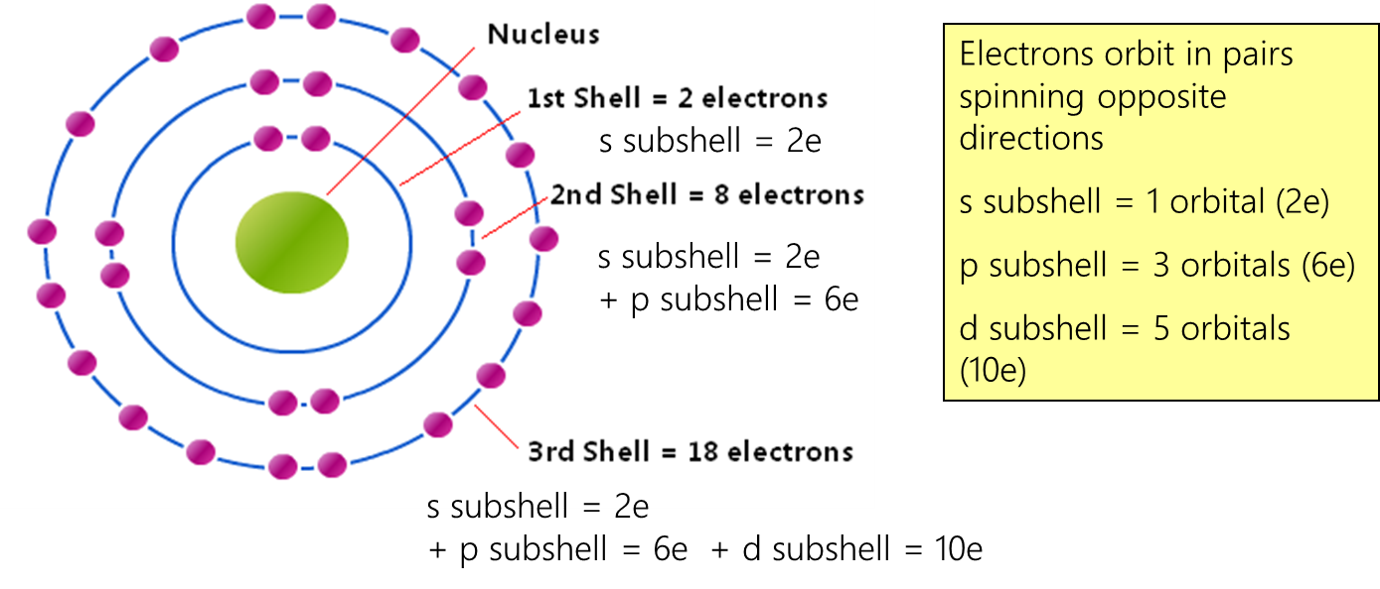
Electrons
1. A Cosmic Dance, Not a Clock
Alright, let's tackle a question that might've kept you up at night (or maybe just popped into your head during a particularly boring meeting): do electrons zip around atoms clockwise or anticlockwise? Well, the slightly mind-bending answer is—it's not quite that simple. Imagine trying to describe the way a honeybee flits around a flower garden. "Clockwise" or "anticlockwise" barely scratches the surface of its chaotic, buzzing path, right?
Electrons aren't tiny planets neatly orbiting a sun. They exist in what we call "orbitals," which are more like probability zones. Think of it as a fuzzy, 3D map showing where an electron might be at any given moment. This is quantum mechanics we're talking about, so prepare for things to get a little weird. Describing their movement with a simple "clockwise" or "anticlockwise" just doesn't cut it. They are not revolving in the way planets revolve around the sun. The concept of 'spin' is important here.
In reality, the motion of an electron is more complex and is associated with its intrinsic angular momentum, which we call "spin." This spin is quantized, meaning it can only take on certain discrete values. The electron's spin is described as either "spin up" or "spin down," which can be loosely associated with clockwise or anticlockwise rotations, but that's more of an analogy than a literal description. Don't get hung up on trying to visualize it as a tiny ball spinning. The math works out, even if our brains struggle to picture it perfectly.
So, rather than picturing neat, predictable orbits, think of electrons as existing in a cloud of probabilities, their behavior governed by the strange and wonderful rules of quantum mechanics. It's less about which direction they're "spinning" and more about the properties they possess that dictate how they interact with other particles. In short, the question itself is a bit misleading because it applies macroscopic thinking to the subatomic world.

Spin Doctors
2. Not Really Spinning, But Close Enough
Okay, so we've established that electrons aren't literally spinning like tops. But the concept of "spin" is crucial to understanding their behavior. Think of it as an intrinsic property, like mass or charge. It's just something electrons have. This spin creates a tiny magnetic field, almost like a mini-magnet is attached to each electron. This is important, because many technologies, like MRI machines, rely on this magnetic property to work.
The two spin states, "spin up" and "spin down," are often represented as +1/2 and -1/2. Again, these aren't directions in the conventional sense. They represent the direction of the magnetic moment associated with the spin. When an electron is placed in a magnetic field, its spin will align either with or against the field. This alignment affects the electron's energy and behavior.
Why is spin important? Well, it's fundamental to the structure of atoms and molecules. Pauli Exclusion Principle, a bedrock of quantum mechanics, states that no two electrons in an atom can have the same set of quantum numbers. This includes spin! So, if two electrons occupy the same orbital, they must have opposite spins. This principle is responsible for the diversity of elements and the way they bond together to form all the matter around us.
In a nutshell, the concept of spin, though abstract, governs how electrons behave. It is important to remember that it's not necessarily about the motion or direction of the electron itself, but rather an intrinsic quantum property that influences its magnetic moment and behavior. It's the key to the chemical behavior of elements, from the simplest hydrogen atom to complex organic molecules.
The Quantum Quandary
3. Beyond Our Everyday Intuition
The reason the "clockwise or anticlockwise" question is so tricky stems from the inherent weirdness of quantum mechanics. Our everyday experience is rooted in classical physics, which deals with objects we can see and touch. But at the subatomic level, things operate according to completely different rules.
In the quantum world, particles can exhibit wave-like properties and exist in multiple states simultaneously (superposition). Their properties are often probabilistic rather than definite. It's like saying a coin is both heads and tails until you actually flip it. Similarly, an electron's "position" or "direction" is not fixed until it's measured or interacts with something else. This is why it's more accurate to describe electrons as existing in orbitals, which are regions of probability, rather than following fixed paths. And also remember that observing the act of measuring it, changes it.
Another complicating factor is Heisenberg's Uncertainty Principle, which states that we can't know both the position and momentum of a particle with perfect accuracy. The more precisely we know one, the less precisely we know the other. So, even if we could somehow peek at an electron's "direction," the act of measuring it would inevitably change its momentum and render the measurement meaningless. You can't sneak a peek without disturbing the electron's delicate quantum state.
So, don't feel bad if you find it hard to wrap your head around. Quantum mechanics is notoriously counterintuitive. It challenges our classical notions of how the world works. Embracing the uncertainty and accepting that some aspects of the subatomic world are simply beyond our ability to fully visualize is part of the journey. It is the way of physics.

Applications
4. Spin's Real-World Impact
Even though electron spin is an abstract concept, it has very real and practical applications. Spin-based electronics, or "spintronics," is a rapidly growing field that leverages the spin of electrons to create new and improved electronic devices. These devices are generally faster, smaller, and more energy-efficient than traditional electronics.
For example, hard drives use giant magnetoresistance (GMR) readers that rely on the spin of electrons to read data. Spintronic materials allow these readers to be much smaller and more sensitive, leading to higher storage densities. Future spintronic devices could include spin-based transistors and memory chips, offering significant performance improvements.
The study of electron spin also plays a crucial role in developing new materials with unique properties. For example, topological insulators are materials that conduct electricity only on their surface, with the bulk of the material acting as an insulator. These materials have potential applications in quantum computing and other advanced technologies. The control of electron spin is also used for developing Quantum computers.
Beyond electronics, electron spin is also essential in understanding chemical reactions and the behavior of molecules. Many chemical reactions are spin-dependent, meaning that the spins of the electrons involved can affect the reaction rate and outcome. This understanding is critical for designing new catalysts and developing more efficient chemical processes. You can see electron spin impacts us in many ways we take for granted.

Quantum Numbers Over 3,097 RoyaltyFree Licensable Stock Vectors
FAQ
5. Your Burning Questions Answered
Q: So, are electrons actually spinning, even if it's not like a top?
A: It's best to think of "spin" as an intrinsic property, like charge, rather than a physical rotation. The term is historical, and it's a bit misleading. It's more accurate to say that electrons possess angular momentum, which is quantized and can be described as "spin up" or "spin down."Q: If electrons aren't orbiting in a neat path, why do we sometimes see diagrams with electrons drawn in circles around the nucleus?
A: Those diagrams are simplified models that are useful for basic understanding. However, they don't accurately represent the true behavior of electrons. In reality, electrons exist in orbitals, which are 3D regions of probability. These models provide a simple representation of the atom, suitable for introductory physics and chemistry, but are not exact.Q: Could we ever build a device that actually "sees" an electron's spin?
A: We can't directly "see" an electron's spin in the way we see a spinning top. However, we can measure its effects using sophisticated instruments. Devices like spin polarimeters can measure the polarization of electrons, which is related to their spin state. Also the measurement of the polarization itself changes the electron properties.
